The electrons of an atom are typically divided into two categories: valence and core electrons. Valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy levels. This difference greatly influences the role of the two types of electrons in a chemical reaction. Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom.
The electron configuration of a oxygen atom is
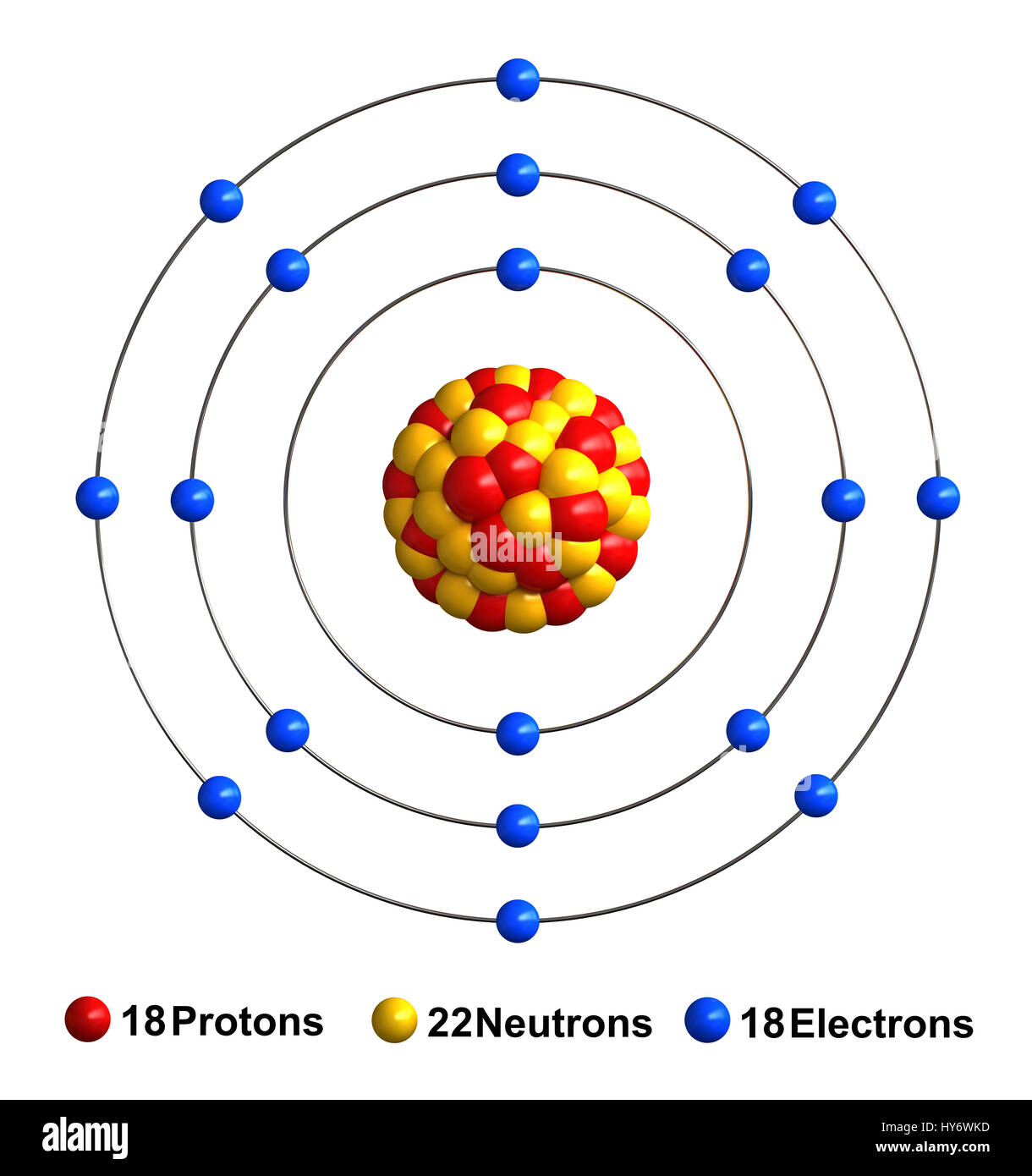
Name: Argon Symbol: Ar Atomic Number: 18 Atomic Mass: 39.9 Number of Protons/Electrons: 18 Number of Neutrons: 22 Classification: Noble Gases Discovery: 1894 Discoverer: Sir William Ramsay. Argon is a noble gas with an electron configuration of N e3s23p6. So, it has 3 electron shells, and that will be its valence shell. In the third shell, it holds a total of 2 + 6 = 8 electrons, so it has 8 valence electrons. The complete octet (eight electrons) in the outer atomic shell makes argon stable and resistant to bonding with other elements. Its triple point temperature of 83.8058 K is a defining fixed point in the International Temperature Scale of 1990. Argon is extracted industrially by the fractional distillation of liquid air.
[ce{O}: ,1s^22s^22p^4 label{1}]
which may be shorted
[ce{O}:, [He]2s^2 2p^4 label{2}]
where the ([He]) stands for the configuration of helium ((1s^2)). Similarly, the configuration of calcium with 20 electrons can be written
The number of electrons. In the outer shell of all the elements in a group is the same as the group number. (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn) - have full outer shells. So if we find are gone on the periodic table, we find that it's on the very right hand side, and the atomic number for are gone is 18 so argon has 18 protons and 18 electrons The 2nd 1 is manganese. Manganese is in the in the center section, the transition metals near that, the top kind of.
[ce{Ca}:, [Ar]4s^2 label{3}]
where the ([Ar]) stands for the configuration of argon ((1s^22s^22p^6 3s^2 3p^6)). Electronic configurations that are the same as noble gases are very stable since they have a full octet (except helium with a full 1s orbital).
The (1s) electrons in oxygen do not participate in bonding (i.e., chemistry) and are called core electrons. The valence electrons (i.e., the (2s^22p^4) part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation (ref{3})), the electrons in the argon-like closed shell are the core electrons and the the two electrons in the 4s orbital are valence electrons.
Example (PageIndex{1}): Cobalt
What are the core and valence electrons in cobalt?
Solution
Fl studio for mac beta. Start by writing the electron configuration of cobalt with 27 electrons:
[1s^22s^22p^63s^23p^64s^23d^7 nonumber]
However, argon has the electronic structure (1s^22s^22p^23s^23p^6), so we can rewrite the configuration as
[[Ar]4s^23d^7 nonumber]
The two electrons in the (4s) orbital and the seven electrons in the (3d) are the valence electrons: all others are core electrons.
The periodicity of valance electrons can be seen in the Periodic Table. Basically, the periodicity is only applied to the main group elements, while in transition metals, rules are complex.
The core electrons remain the same in the increase of group numbers in the main group elements. On the other hand, the valance electrons increase by one from left to right of a main period, and remain the same down the column of a main group. This evolution gives periodical change in property of a period, and similar chemical property of a group, which is called periodical trend. The number of valence electrons in a main period is the same as its group number. The table below shows this rule clearly.
Under construction
Figure 1: 1A + 2A are metals. 3A to 8A are non-metals.
However, this periodicity cannot be applied to the transition group, which is more complicated than that of the main group. Although the outermost electrons can be easily determined, the apparent valence electrons considered in chemical reactivity are complex and fluctuated. Electrons going into d sublevel can play either a role of valence electrons or shielding electrons. So there is not always a certain number of apparent valence electrons. The number of apparent valence electrons for the first transition metal period is shown in the table below.
Under construction
Figure 2: Valence electrons for transition metals.
Relationship with Chemical Reactivity
The chemical reactivity of an atom is mainly determined by valence electrons. Atoms which have a complete shell of valence electrons tend to be chemically inert. Atoms with one or two valence electrons are highly reactive. This phenomenon can be explained by Hund's rule, which states that orbitals that are empty, half-full, or full are more stable than those that are not. For example, Ne is chemically inert because it has two valence electrons that fill its outermost shell which makes it stable compared to atoms such as Al, which has three valence electrons, but its valence electrons does not fill its outermost shell.
Although core electrons do not take part in chemical bonding, they play a role in determining the chemical reactivity of an atom. This influence is generally due to the effect it has on valence electrons. The effect can be observed from the gradual change of chemical reactivity in a group. As you go down a group, more shells are occupied by electrons, which increases the size of the atom. The more core electron shells an atom has, the larger the size of the atom, and the farther the valence electrons are from the nucleus, thus the valence electrons will experience less effective nuclear charge and will be easily lost. For example, (ce{Na}) and (ce{K}) can both react with water, but K has a more radical reaction because it has more shells of core electrons which makes the valence electron in its outermost orbital much easier to lose than the valence electron of Na.
References
- Miessler, Gary L., and Donald A. Tarr. Inorganic Chemistry. Upper Saddle River, NJ: Pearson Prentice Hall, 2010. Print.
- Brown, Ian David. The Chemical Bond in Inorganic Chemistry the Bond Valence Model. Oxford: Oxford UP, 2006. Print.
2
The noble gases are the chemical elements in group 18 of the periodic table.
They are the most stable due to having the maximum number of valence electrons their outer shell can hold.
Mac for small business. Therefore, they rarely react with other elements since they are already stable.
Other characteristics of the noble gases are that they all conduct electricity, fluoresce, are odorless and colorless, and are used in many conditions when a stable element is needed to maintain a safe and constant environment.
This chemical series contains helium, neon, argon, krypton, xenon, and radon.
The noble gases were previously referred to as inert gases, but this term is not strictly accurate because several of them do take part in chemical reactions.
2
Scientists Announce a 'Nuclear' Periodic Table
New Periodic Table of Droplets Could Help Solve Crimes
Superheroes, Foods and Apps Bring a Modern Twist to the Periodic Table
Noble Metal Clusters Can Enhance Performance of Catalysts and Save Resources

Discovery of 'Periodic Tables' for Molecules
Experimental Tests of Relativistic Chemistry Will Update the Periodic Table
New Catalyst Can Make Natural Gas Burn Cleaner
Creating Attraction Between Molecules Deep in the Periodic Table
Methane to Syngas Catalyst: Two for the Price of One
Quantum Wave in Helium Dimer Filmed for the First Time
- RELATED TERMS
- Xenon
- Krypton
- Radon
- Helium
- Neon
- Argon
Inspired by Nature, the Research to Develop a New Load-Bearing Material
Synthetic Gelatin-Like Material Mimics Lobster Underbelly's Stretch and Strength
Quantum Steering for More Precise Measurements
How Many Protons In Argon
Scientists Uncover Structure of Light-Driven Enzyme With Potential Biofuel Applications
3D Printed Models Provide Clearer Understanding of Ground Motion
High School Junior's Consumer Seismometer Delivers Low-Cost Earthquake Early Warning
DeepShake Uses Machine Learning to Rapidly Estimate Earthquake Shaking Intensity
SMART Breakthrough in Materials Discovery Enables 'Twistronics' for Bulk Systems
Beyond Space-Age Tech: Hybrid Material Moves Next-Generation Transport Fuel Cells Closer
Ten Ways to Ensure Bees Benefit from the Solar Power Boom
Number Of Electrons In Argon 40
