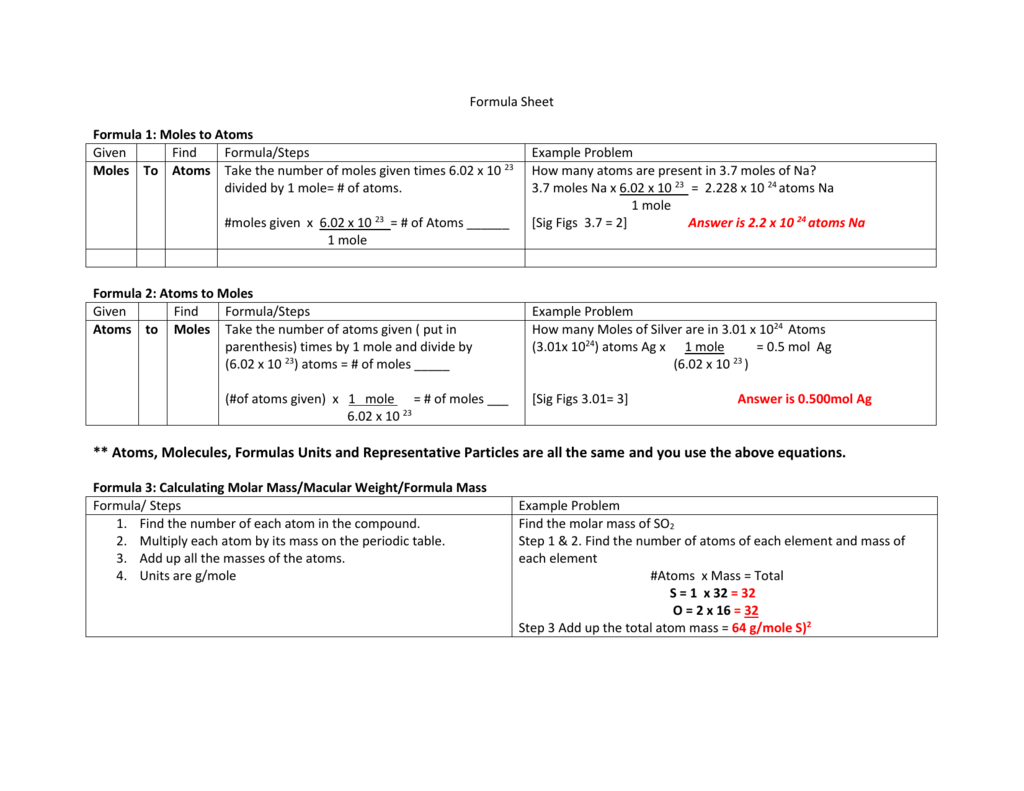
Avogadro’s Number and the Mole
+Moles++Atoms%2FMolecules.jpg)
- A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms. 2 Sulfuric acid has the chemical formula H 2 SO 4. A certain quantity of sulfuric acid contains 4.89 × 10 25 atoms of oxygen.
- The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol.' ›› Definition: Atom This site uses an exact value of 6.0221415 x 10 23 for Avogadro's number.
Atoms to Grams Formula. The following equation is used to calculate the total number of grams from atoms. Where G is grams; A is the total number of atoms; AAM is the average atomic mass of the atoms; Atoms to Grams Definition. Converting atoms to grams involves multiply the total number of atoms by the average atomic mass of the.
The mole is represented by Avogadro’s number, which is 6.022×1023 atoms or molecules per mol.
Learning Objectives
Define and memorize Avogadro’s number
Key Takeaways
Key Points
- The mole allows scientists to calculate the number of elementary entities (usually atoms or molecules ) in a certain mass of a given substance.
- Avogadro’s number is an absolute number: there are 6.022×1023 elementary entities in 1 mole. This can also be written as 6.022×1023 mol-1.
- The mass of one mole of a substance is equal to that substance’s molecular weight. For example, the mean molecular weight of water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams.
Key Terms
- mole: The amount of substance of a system that contains as many elementary entities as there are atoms in 12 g of carbon-12.
The chemical changes observed in any reaction involve the rearrangement of billions of atoms. It is impractical to try to count or visualize all these atoms, but scientists need some way to refer to the entire quantity. They also need a way to compare these numbers and relate them to the weights of the substances, which they can measure and observe. The solution is the concept of the mole, which is very important in quantitative chemistry.
Avogadro’s Number
Amedeo Avogadro: Amedeo Avogadro is credited with the idea that the number of entities (usually atoms or molecules) in a substance is proportional to its physical mass.
Amadeo Avogadro first proposed that the volume of a gas at a given pressure and temperature is proportional to the number of atoms or molecules, regardless of the type of gas. Although he did not determine the exact proportion, he is credited for the idea.
Avogadro’s number is a proportion that relates molar mass on an atomic scale to physical mass on a human scale. Avogadro’s number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022×1023 mol-1 and is expressed as the symbol NA.
Avogadro’s number is a similar concept to that of a dozen or a gross. A dozen molecules is 12 molecules. A gross of molecules is 144 molecules. Avogadro’s number is 6.022×1023 molecules. With Avogadro’s number, scientists can discuss and compare very large numbers, which is useful because substances in everyday quantities contain very large numbers of atoms and molecules.
The Mole
The mole (abbreviated mol) is the SI measure of quantity of a “chemical entity,” such as atoms, electrons, or protons. It is defined as the amount of a substance that contains as many particles as there are atoms in 12 grams of pure carbon-12. So, 1 mol contains 6.022×1023 elementary entities of the substance.
Chemical Computations with Avogadro’s Number and the Mole
Avogadro’s number is fundamental to understanding both the makeup of molecules and their interactions and combinations. For example, since one atom of oxygen will combine with two atoms of hydrogen to create one molecule of water (H2O), one mole of oxygen (6.022×1023 of O atoms) will combine with two moles of hydrogen (2 × 6.022×1023 of H atoms) to make one mole of H2O.
Another property of Avogadro’s number is that the mass of one mole of a substance is equal to that substance’s molecular weight. For example, the mean molecular weight of water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams. This property simplifies many chemical computations.
If you have 1.25 grams of a molecule with molecular weight of 134.1 g/mol, how many moles of that molecule do you have?
[latex]1.25text{ g} times frac{ 1 text{ mole}}{134.1text{ g}}=0.0093 text{ moles}[/latex]
The Mole, Avogadro: This video introduces counting by mass, the mole, and how it relates to atomic mass units (AMU) and Avogadro’s number.
Converting between Moles and Atoms
By understanding the relationship between moles and Avogadro’s number, scientists can convert between number of moles and number of atoms.
Learning Objectives
Convert between the number of moles and the number of atoms in a given substance using Avagadro’s number
Key Takeaways
Key Points
- Avogadro’s number is a very important relationship to remember: 1 mole = [latex]6.022times10^{23}[/latex] atoms, molecules, protons, etc.
- To convert from moles to atoms, multiply the molar amount by Avogadro’s number.
- To convert from atoms to moles, divide the atom amount by Avogadro’s number (or multiply by its reciprocal).
Key Terms
- mole: The amount of substance of a system that contains as many elementary entities as there are atoms in 12 g of carbon-12.
- Avogadro’s number: The number of atoms present in 12 g of carbon-12, which is [latex]6.022times10^{23}[/latex] and the number of elementary entities (atoms or molecules) comprising one mole of a given substance.
Moles and Atoms
As introduced in the previous concept, the mole can be used to relate masses of substances to the quantity of atoms therein. This is an easy way of determining how much of one substance can react with a given amount of another substance.
From moles of a substance, one can also find the number of atoms in a sample and vice versa. The bridge between atoms and moles is Avogadro’s number, 6.022×1023.
Avogadro’s number is typically dimensionless, but when it defines the mole, it can be expressed as 6.022×1023 elementary entities/mol. This form shows the role of Avogadro’s number as a conversion factor between the number of entities and the number of moles. Therefore, given the relationship 1 mol = 6.022 x 1023 atoms, converting between moles and atoms of a substance becomes a simple dimensional analysis problem.
Converting Moles to Atoms
Given a known number of moles (x), one can find the number of atoms (y) in this molar quantity by multiplying it by Avogadro’s number:
[latex]x text{ moles}cdotfrac {6.022times10^{23}text{atoms}}{1text{ mole}} = ytext{ atoms}[/latex]
For example, if scientists want to know how may atoms are in six moles of sodium (x = 6), they could solve:
[latex]6text{ moles}cdotfrac {6.022times 10^{23}text{ atoms}}{1text{ mole}} = 3.61times 10^{24}text{ atoms}[/latex]
Note that the solution is independent of whether the element is sodium or otherwise.
Converting Atoms to Moles
Reversing the calculation above, it is possible to convert a number of atoms to a molar quantity by dividing it by Avogadro’s number:
[latex]frac{{xtext{ atoms}}}{{6.022times 10^{23} frac{text{atoms}}{1text{ mole}}}}= ytext{ moles}[/latex]
This can be written without a fraction in the denominator by multiplying the number of atoms by the reciprocal of Avogadro’s number:
[latex]x text{ atoms}cdotfrac{1text{ mole}}{6.022times 10^{23}text{ atoms}} = y text{ moles}[/latex]
For example, if scientists know there are [latex]3.5 cdot 10^{24} [/latex]atoms in a sample, they can calculate the number of moles this quantity represents:
[latex]3.5times 10^{24}text{ atoms}cdotfrac{1text{ mole}}{6.022times 10^{23} text{ atoms}} = 5.81text{ moles}[/latex]
Molar Mass of Compounds
The molar mass of a particular substance is the mass of one mole of that substance.
Learning Objectives
Calculate the molar mass of an element or compound
Key Takeaways
Key Points
- The molar mass is the mass of a given chemical element or chemical compound (g) divided by the amount of substance (mol).
- The molar mass of a compound can be calculated by adding the standard atomic masses (in g/mol) of the constituent atoms.
- Molar mass serves as a bridge between the mass of a material and the number of moles since it is not possible to measure the number of moles directly.
Key Terms
Atoms To Moles To Grams Calculator
- molar mass: The mass of a given substance (chemical element or chemical compound in g) divided by its amount of substance (mol).
- mole: The amount of substance of a system that contains as many elementary entities as there are atoms in 12 g of carbon-12.
Measuring Mass in Chemistry
Chemists can measure a quantity of matter using mass, but in chemical reactions it is often important to consider the number of atoms of each element present in each sample. Even the smallest quantity of a substance will contain billions of atoms, so chemists generally use the mole as the unit for the amount of substance.
One mole (abbreviated mol) is equal to the number of atoms in 12 grams of carbon-12; this number is referred to as Avogadro’s number and has been measured as approximately 6.022 x 1023. In other words, a mole is the amount of substance that contains as many entities (atoms, or other particles) as there are atoms in 12 grams of pure carbon-12.
amu vs. g/mol
Each ion, or atom, has a particular mass; similarly, each mole of a given pure substance also has a definite mass. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units (amu) or in grams per mole (g/mol). Although mass can be expressed as both amu and g/mol, g/mol is the most useful system of units for laboratory chemistry.
Calculating Molar Mass
Molar mass is the mass of a given substance divided by the amount of that substance, measured in g/mol. For example, the atomic mass of titanium is 47.88 amu or 47.88 g/mol. In 47.88 grams of titanium, there is one mole, or 6.022 x 1023 titanium atoms.
The characteristic molar mass of an element is simply the atomic mass in g/mol. However, molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant (1 g/mol). To calculate the molar mass of a compound with multiple atoms, sum all the atomic mass of the constituent atoms.
For example, the molar mass of NaCl can be calculated for finding the atomic mass of sodium (22.99 g/mol) and the atomic mass of chlorine (35.45 g/mol) and combining them. The molar mass of NaCl is 58.44 g/mol.
Molar Mass Calculations – YouTube: This video shows how to calculate the molar mass for several compounds using their chemical formulas.
Converting between Mass and Number of Moles
A substance’s molar mass can be used to convert between the mass of the substance and the number of moles in that substance.
Learning Objectives
Convert between the mass and the number of moles, and the number of atoms, in a given sample of compound
Key Takeaways
Key Points
- The molar mass of a compound is equal to the sum of the atomic masses of its constituent atoms in g/mol.
- Although there is no physical way of measuring the number of moles of a compound, we can relate its mass to the number of moles by using the compound’s molar mass as a direct conversion factor.
- To convert between mass and number of moles, you can use the molar mass of the substance. Then, you can use Avogadro’s number to convert the number of moles to number of atoms.
Key Terms
- molar mass: The mass of a given substance (chemical element or chemical compound) divided by its amount of substance (mol), in g/mol.
- dimensional analysis: The analysis of the relationships between different physical quantities by identifying their fundamental dimensions (such as length, mass, time, and electric charge) and units of measure (such as miles vs. kilometers, or pounds vs. kilograms vs. grams) and tracking these dimensions as calculations or comparisons are performed.
- mole: The amount of substance that contains as many elementary entities as there are atoms in 12 g of carbon-12.
Chemists generally use the mole as the unit for the number of atoms or molecules of a material. One mole (abbreviated mol) is equal to 6.022×1023 molecular entities (Avogadro’s number), and each element has a different molar mass depending on the weight of 6.022×1023 of its atoms (1 mole). The molar mass of any element can be determined by finding the atomic mass of the element on the periodic table. For example, if the atomic mass of sulfer (S) is 32.066 amu, then its molar mass is 32.066 g/mol.
By recognizing the relationship between the molar mass (g/mol), moles (mol), and particles, scientists can use dimensional analysis convert between mass, number of moles and number of atoms very easily.
Converting between mass, moles, and particles: This flowchart illustrates the relationships between mass, moles, and particles. These relationships can be used to convert between units.
Determining the Molar Mass of a Compound
In a compound of NaOH, the molar mass of Na alone is 23 g/mol, the molar mass of O is 16 g/mol, and H is 1 g/mol. What is the molar mass of NaOH?
[latex]text{Na}+text{O}+text{H}=text{NaOH}[/latex]
[latex]23 space text{g/mol} +16 space text{g/mol}+ 1 space text{g/mol} = 40 space text{g/mol}[/latex]
The molar mass of the compound NaOH is 40 g/mol.
Converting Mass to Number of Moles
How many moles of NaOH are present in 90 g of NaOH?
Since the molar mass of NaOH is 40 g/mol, we can divide the 90 g of NaOH by the molar mass (40 g/mol) to find the moles of NaOH. This the same as multiplying by the reciprocal of 40 g/mol.
If the equation is arranged correctly, the mass units (g) cancel out and leave moles as the unit.
[latex]90text{ g}space text{NaOH} times frac{1 text{ mol}}{40text{ g}} = 2.25 space text{mol NaOH}[/latex]
There are 2.25 moles of NaOH in 90g of NaOH.
Converting Between Mass, Number of Moles, and Number of Atoms
How many moles and how many atoms are contained in 10.0 g of nickel?
According to the periodic table, the atomic mass of nickel (Ni) is 58.69 amu, which means that the molar mass of nickel is 58.69 g/mol. Therefore, we can divide 10.0 g of Ni by the molar mass of Ni to find the number of moles present.
Using dimensional analysis, it is possible to determine that:
[latex]10text{ g Ni}times frac{1text{ mol Ni}}{58.69text{ g Ni}} = 0.170text{ mol Ni}[/latex]
To determine the number of atoms, convert the moles of Ni to atoms using Avogadro’s number:
[latex]0.170text{ moles Ni}timesfrac {6.022times10^{23}text{ atoms Ni}}{1text{ mol Ni}} = 1.02times10^{23}text{ atoms Ni}[/latex]
Given a sample’s mass and number of moles in that sample, it is also possible to calculate the sample’s molecular mass by dividing the mass by the number of moles to calculate g/mol.
What is the molar mass of methane (CH4) if there are 0.623 moles in a 10.0g sample?
[latex]frac{10.0 text{ g CH}_4}{0.623 text{ mol CH}_4} = 16.05 text{ g/mol CH}_4 [/latex]
The molar mass of CH4 is 16.05 g/mol.
Even with a good word-processing program, having to click on an icon to get a superscript, and then remembering to click off after you type the number can be a real hassle. If we did not know of moles, and instead just knew of numbers of atoms or molecules (big numbers that require lots of superscripts), life would be much more complicated; we would certainly make more typing errors.
Atoms To Moles To Grams
Conversions Between Moles and Atoms
Conversions Between Moles and Number of Particles
Using our unit conversion techniques, we can use the mole label to convert back and forth between the number of particles and moles.
Example (PageIndex{1})
The element carbon exists in two primary forms: graphite and diamond. How many moles of carbon atoms is (4.72 times 10^{24}) atoms of carbon?
Solution
Step 1: List the known quantities and plan the problem.
Known
- number of (ce{C}) atoms (= 4.72 times 10^{24})
- (1) mole (= 6.02 times 10^{23}) atoms
Unknown
- (4.72 times 10^{24} = ?) atoms (ce{C})
One conversion factor will allow us to convert from the number of (ce{C}) atoms to moles of (ce{C}) atoms.
Step 2: Calculate.
[4.72 times 10^{24} : text{atoms} : ce{C} times frac{1 : text{mol} : ce{C}}{6.02 times 10^{23} : text{atoms} : ce{C}} = 7.84 : text{mol} : ce{C}]
Step 3: Think about your result.
The given number of carbon atoms was greater than Avogadro's number, so the number of moles of (ce{C}) atoms is greater than 1 mole. Since Avogadro's number is a measured quantity with three significant figures, the result of the calculation is rounded to three significant figures.
Suppose that you want to know how many hydrogen atoms are in a mole of water molecules. First, you need to know the chemical formula for water, which is (ce{H_2O}). There are two atoms of hydrogen in each molecule of water. How many atoms of hydrogen are in two water molecules? There are (2 times 2 = 4) hydrogen atoms. How about in a dozen? In that case, a dozen is 12; so (12 times 2 = 24) hydrogen atoms in a dozen water molecules. To get the answers (4 and 24), you multiply the given number of molecules by two atoms of hydrogen per molecule. So, to find the number of hydrogen atoms in a mole of water molecules, the problem can be solved using conversion factors:
[1 : text{mol} : ce{H_2O} times frac{6.02 times 10^{23} : text{molecules} : ce{H_2O}}{1 : text{mol} : ce{H_2O}} times frac{2 : text{atoms} : ce{H}}{1 : text{molecule} : ce{H_2O}} = 1.20 times 10^{24} : text{atoms} : ce{H}]
The first conversion factor converts from moles of particles to the number of particles. The second conversion factor reflects the number of atoms contained within each molecule.
/Amedeo-Avogadro-58de9ad03df78c5162bef126.jpg)
Example (PageIndex{2})
Sulfuric acid has the chemical formula (ce{H_2SO_4}). A certain quantity of sulfuric acid contains (4.89 times 10^{25}) atoms of oxygen. How many moles of sulfuric acid is the sample?
Solution:
Step 1: List the known quantities and plan the problem.
Known
- (4.89 times 10^{25} = ce{O}) atoms
- (1) mole (= 6.02 times 10^{23}) molecules (ce{H_2SO_4})
Unknown
- (text{mol}) of (ce{H_2SO_4}) molecules
Two conversion factors will be used. First, convert atoms of oxygen to molecules of sulfuric acid. Then, convert molecules of sulfuric acid to moles of sulfuric acid.
Step 2: Calculate.
[4.89 times 10^{25} : text{atoms} : ce{O} times frac{1 : text{molecule} : ce{H_2SO_4}}{4 : text{atoms} : ce{O}} times frac{1 : text{mol} : ce{H_2SO_4}}{6.02 times 10^{23} : text{molecules} : ce{H_2SO_4}} = 20. : text{mol} : ce{H_2SO_4}]
Step 3: Think about your result.
The original number of oxygen atoms was about 80 times larger than Avogadro's number. Since each sulfuric acid molecule contains 4 oxgyen atoms, there are about 20 moles of sulfuric acid molecules.
Summary
- Methods are described for conversions between moles, atoms, and molecules.
Contributors and Attributions
Atoms To Moles Na
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
